Pilot study of 1650-G. Human Papillomavirus HPV Vaccine.
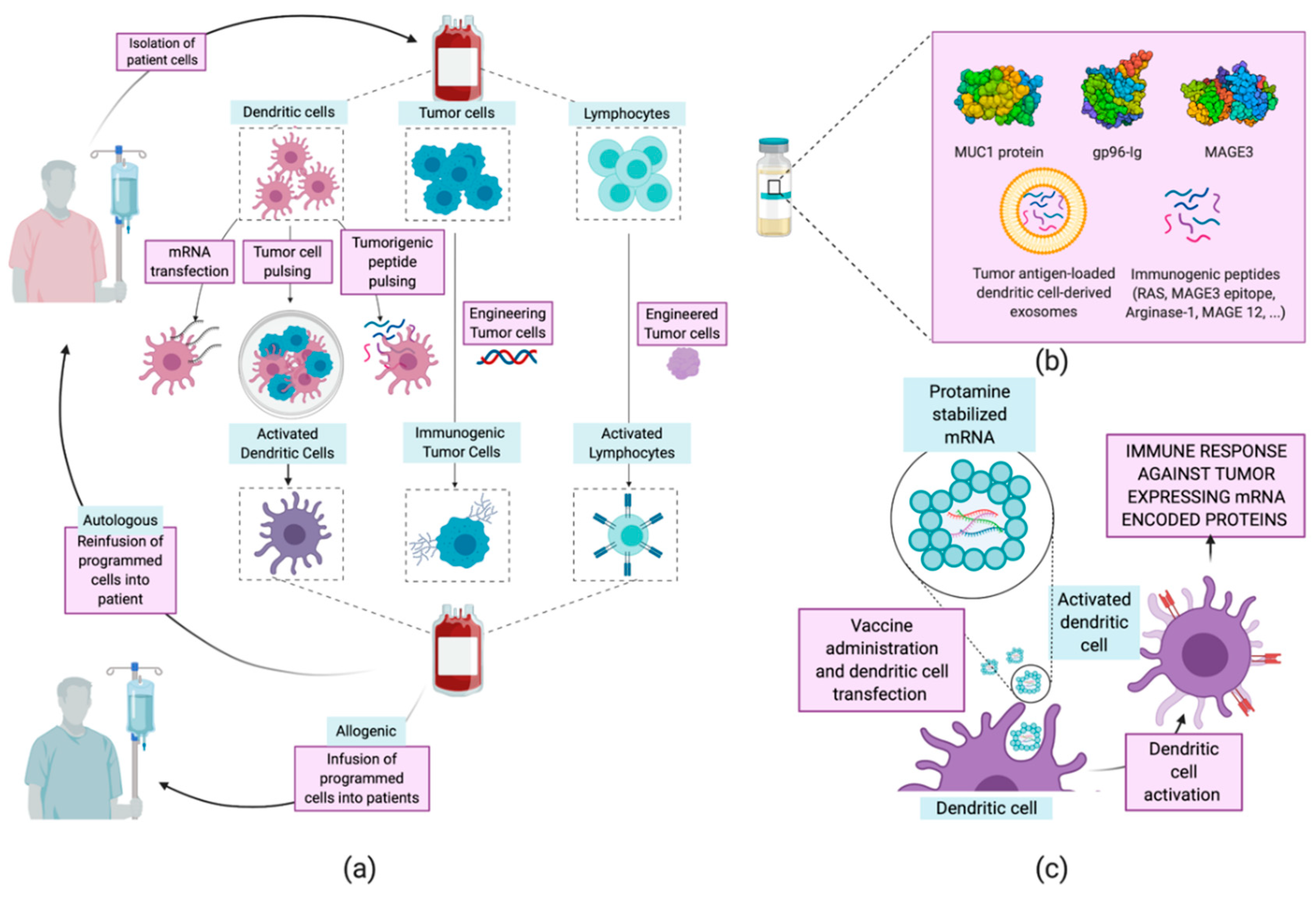
Generation Of Dendritic Cell Based Vaccine Using High Hydrostatic Pressure For Non Small Cell Lung Cancer Immunotherapy
Vaccines that treat existing cancer are known as therapeutic cancer vaccines.
1650 g lung cancer vaccine. Some researchers claim that cancerous cells routinely. So vaccines are mainly available as part of clinical trials. Efforts to develop lung cancer vaccines began more than 20 years ago but have met with disappointment.
These antigens include carcinoembryonic antigen CEA MUC1 guanylyl cyclase C and NY-ESO-1. These findings provide critical foundation for further testing of this simple and comparatively. Patients receive 2 injections of.
But this will only happen if positive results are being shown throughout all phases of testing. Upon administration allogeneic cellular vaccine 1650-G may. This review discusses the most promising vaccination strategies for non-small cell lung cancer including the allogeneic tumor cell vaccine belagenpumatucel-L which is a mixture of 4 allogeneic non-small cell lung cancer cell lines genetically modified to secrete an antisense oligonucleotide to transforming growth factor 2 and 3 other target protein-specific vaccines designed to.
Patients receive 2 injections of 1650-G Vaccine given 4 weeks apart for a total of 52 weeks on study. After being tested on some 1700 patients including 1200 included in an ongoing clinical trial the drug was approved for use in late-stage lung cancer patients. 20 Belagenpumatucel-l an allogeneic tumor cell vaccine that incorporates a TGF- antisense gene was well tolerated and showed a survival advantage in 75 NSCLC patients.
This report describes early clinical experience with vaccine 1650-G an allogeneic cellular vaccine using granulocyte macrophage colony stimulating factor as an adjuvant. Unlike vaccines to protect us from disease cancer treatment vaccines are for people who already have cancer. These types of vaccines are only useful for cancers known to be caused by infections.
Food and Drug Administration. The HPV vaccine protects against the types of HPV that most often cause these cancers. Clinical trials for at least 7 therapeutic vaccines were initiated against nonsmall cell.
Vaccines are a type of immunotherapy. 21 A third vaccine. Researchers are making treatment vaccines that tell the body to attack cells with antigens thought to cause colorectal cancer.
Unlike cancer prevention vaccines cancer treatment vaccines are designed to be used in people who already have cancerthey work against cancer cells not against something that causes cancer. Cancer treatment vaccines are different from the vaccines that work against viruses. What Is a Cancer Vaccine.
1 2004 Vienna Austria -- A novel non small cell lung cancer vaccine that stimulates the immune system to attack tumor cells is helping people with lung cancer live longer researchers say. Since antigen delivered with the DC vaccines also included foreign MHC derived from 1650 a wheal and flare skin reaction or immunologic response measured by IFN- ELISPOT based solely on alloreactivity is possible. Someday we may have a vaccine that prevents all types of cancer especially the more deadly types like lung cancer.
Vaccines to treat cancer. 1650-independent responses noted in five of sixteen individuals might also indicate reactivity to antigens ingested by DCs during culture such as. Cancer vaccines help your bodys immune system recognise and attack cancer cells.
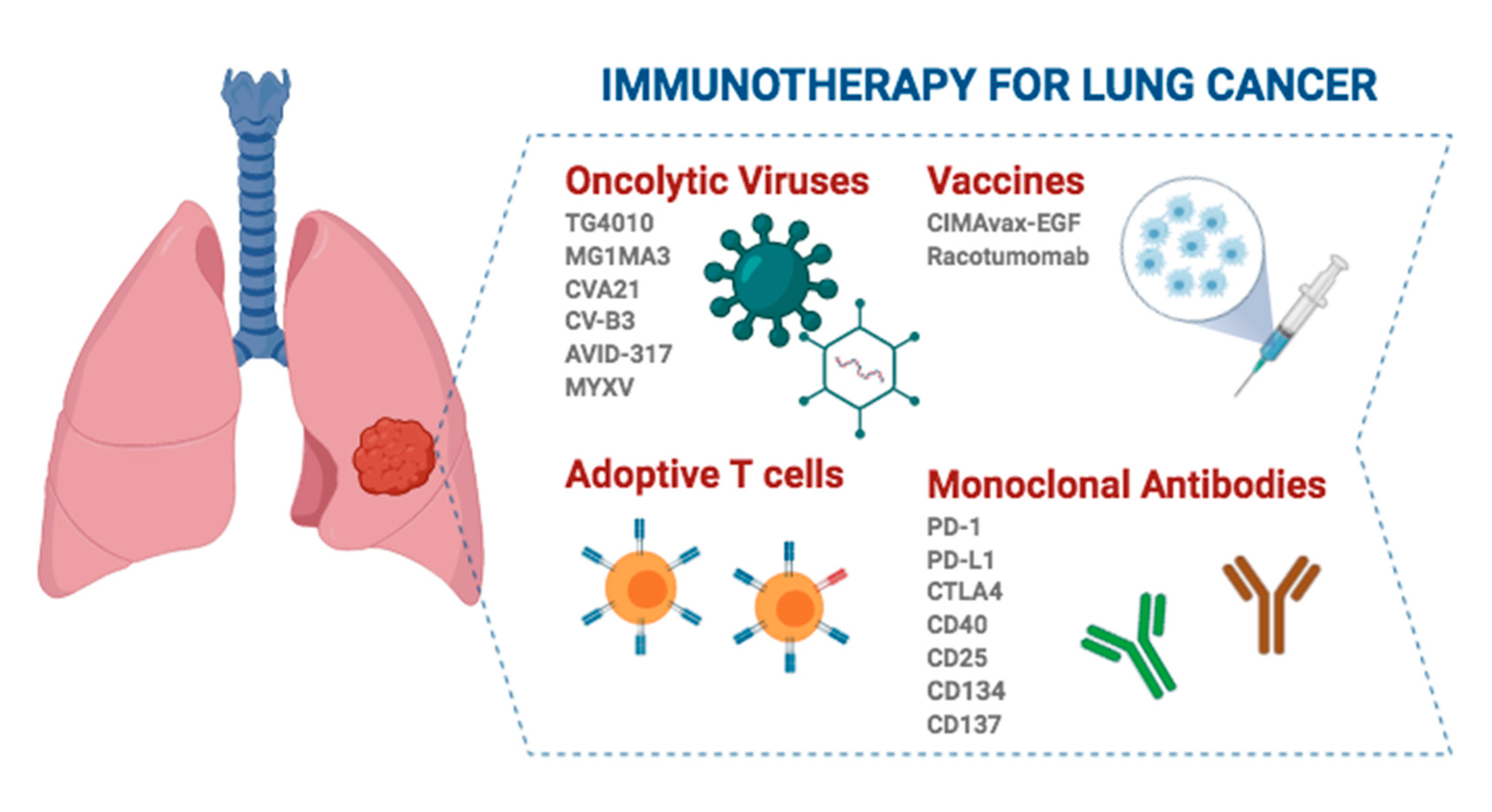
Several peptide gene- and cell-based vaccines are been evaluated in the clinical setting. Treatment vaccines can help the immune system learn to recognize and react to these antigens and destroy cancer. Rather they are a kind of immunotherapy that prompts the bodys immune system to battle the disease in patients with non-small cell lung cancer.
HPV vaccination is recommended for preteens aged 11 to 12 years but can be given starting at age 9. A lung cancer vaccine made from the patients own tumor cells is showing promise. In January the Roswell Park Comprehensive Cancer Center in Buffalo New York launched a clinical trial of Cimavax with Cubas Center of Molecular Immunology which developed the vaccine.
Neither of the vaccines prevents cancer. Our interest in producing and testing 1650-G is to expand on previous work with DC vaccines for lung cancer14 15 Those data show that autologous DCs pulsed with an allogeneic cell line that expresses a complement of tumor antigens 1650 clearly induce immune responses in a percentage of patients performing comparably to if not better than most other solid tumor vaccines. A simplified cellular vaccine for lung cancer.
A cancer vaccine is a vaccine that either treats existing cancer or prevents development of cancer. The idea behind treatment vaccines is that cancer cells contain substances called tumor-associated antigens that are not present in normal cells or if present are at lower levels. 19 The MUC-1based L-BLP25 tested in a similar patient subset showed a survival trend in favor of L-BLP25 in a subgroup of patients with locoregional stage IIIB.
Researchers are testing many cancer vaccines to treat kidney cancer. Research in this area is at an early stage. For now there are only three cancer vaccines approved by the US.
Although no immunotherapy is likely to be a panacea recent data from randomized phase IIB studies offer promise of therapeutic activity in both early and late stage lung cancer. But most cancers including colorectal lung prostate and breast cancers are not thought to be caused by infections. 129 rows 1650-G Arm.
Somemany of the vaccines are autologous being prepared from samples taken from the patient and are specific to that patient. 1650-G Vaccine 6ml injection administered intradermally in the thigh at week 0 and week 4. Data collection and curation is an ongoing process for CDEK - if you notice any information here to be missing or incorrect please let us know.
A pluripotent allogeneic tumor cell vaccine composed of irradiated tumor cells from the non-small cell lung cancer NSCLC cell line 1650 and the immunoadjuvant recombinant granulocyte-macrophage colony stimulating factor GM-CSF 1650-G with potential immunostimulating and antineoplastic activities. If the need for the vaccine is urgent such as the COVID-19 vaccines currently in or having completed Phase III testing and the vaccine is found to be effective the FDA may give preliminary or conditional approval before all the testing is completed. Carcinoma Non-Small-Cell Lung Phase 2 Lung Neoplasms Phase 2.
Some cancers are caused by human papillomavirus HPV a very common sexually transmitted infection.

Intratumoral Injection Of The Seasonal Flu Shot Converts Immunologically Cold Tumors To Hot And Serves As An Immunotherapy For Cancer Pnas

Pilot Study Of 1650 G A Simplified Cellular Vaccine For Lung Cancer Journal Of Thoracic Oncology

Cancers Free Full Text Nanomedicine In Non Small Cell Lung Cancer From Conventional Treatments To Immunotherapy Html
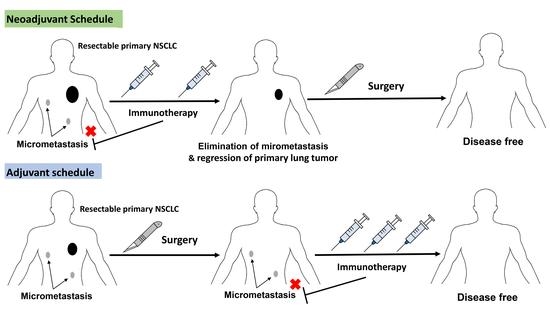
Vaccines Free Full Text Immunotherapy And Vaccination In Surgically Resectable Non Small Cell Lung Cancer Nsclc Html

Cancers Free Full Text Nanomedicine In Non Small Cell Lung Cancer From Conventional Treatments To Immunotherapy Html
Cancers Free Full Text Primary And Acquired Resistance To Immunotherapy In Lung Cancer Unveiling The Mechanisms Underlying Of Immune Checkpoint Blockade Therapy Html

Pdf Generation Of Dendritic Cell Based Vaccine Using High Hydrostatic Pressure For Non Small Cell Lung Cancer Immunotherapy

References In Immunotherapies For Non Small Cell Lung Cancer And Mesothelioma The Lancet Oncology
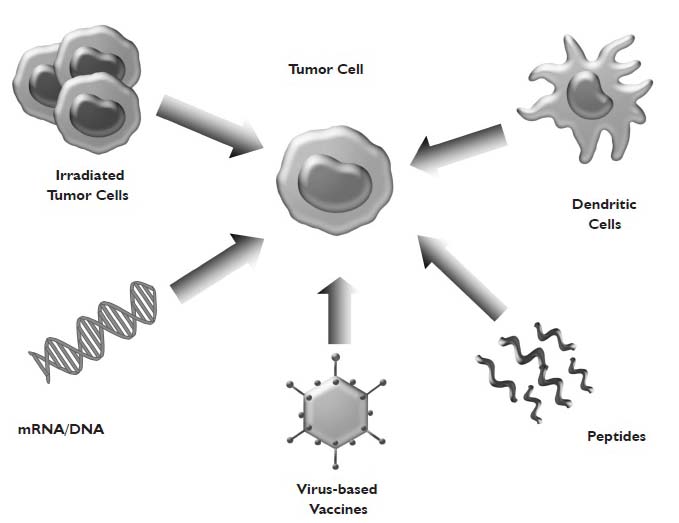
Active Specific Immunotherapy For Non Small Cell Lung Cancer Winter Journal Of Thoracic Disease

Therapeutic Strategies To Remodel Immunologically Cold Tumors Wang 2020 Clinical Amp Translational Immunology Wiley Online Library

Cimavax Lung Cancer Vaccine Roswell Park Comprehensive Cancer Center

Pdf Generation Of Dendritic Cell Based Vaccine Using High Hydrostatic Pressure For Non Small Cell Lung Cancer Immunotherapy
Generation Of Dendritic Cell Based Vaccine Using High Hydrostatic Pressure For Non Small Cell Lung Cancer Immunotherapy
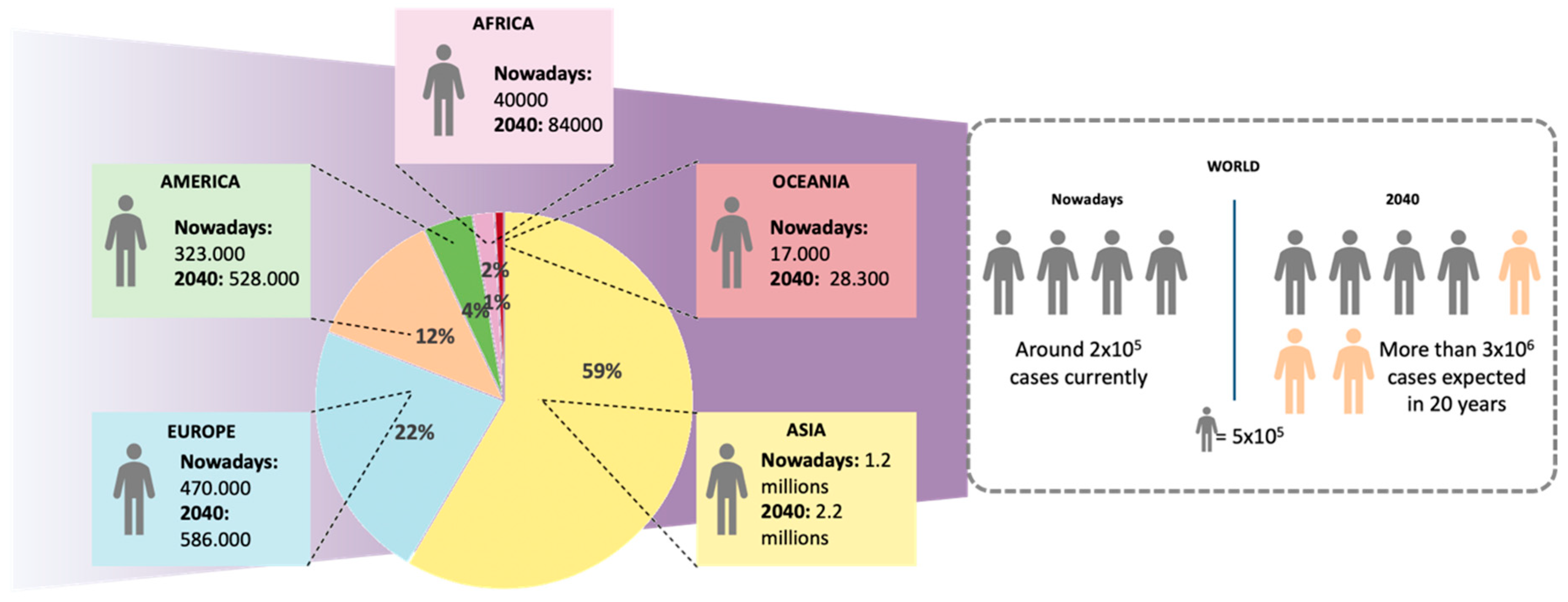
Cancers Free Full Text Nanomedicine In Non Small Cell Lung Cancer From Conventional Treatments To Immunotherapy Html

Principles Of Immunotherapy In Non Small Cell Lung Cancer Thoracic Surgery Clinics
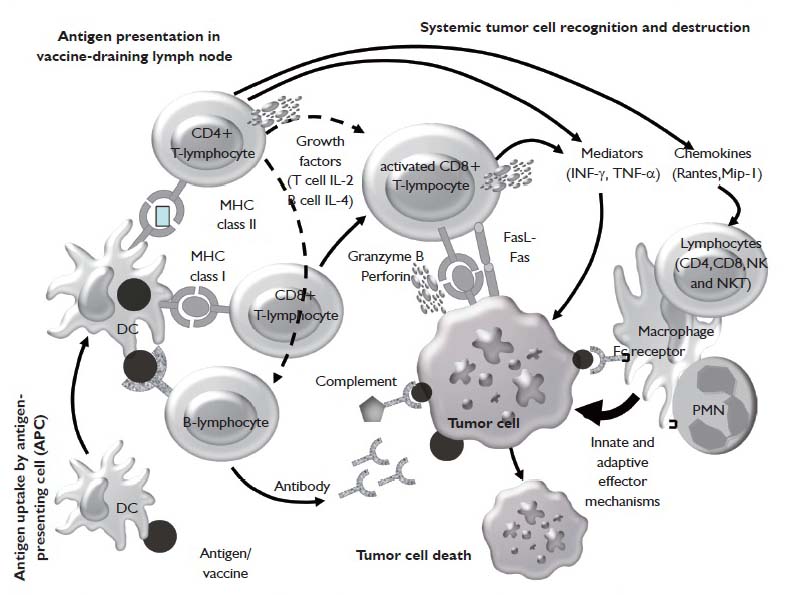
Active Specific Immunotherapy For Non Small Cell Lung Cancer Winter Journal Of Thoracic Disease

Antitumor Effects Of Ipsc Based Cancer Vaccine In Pancreatic Cancer Sciencedirect
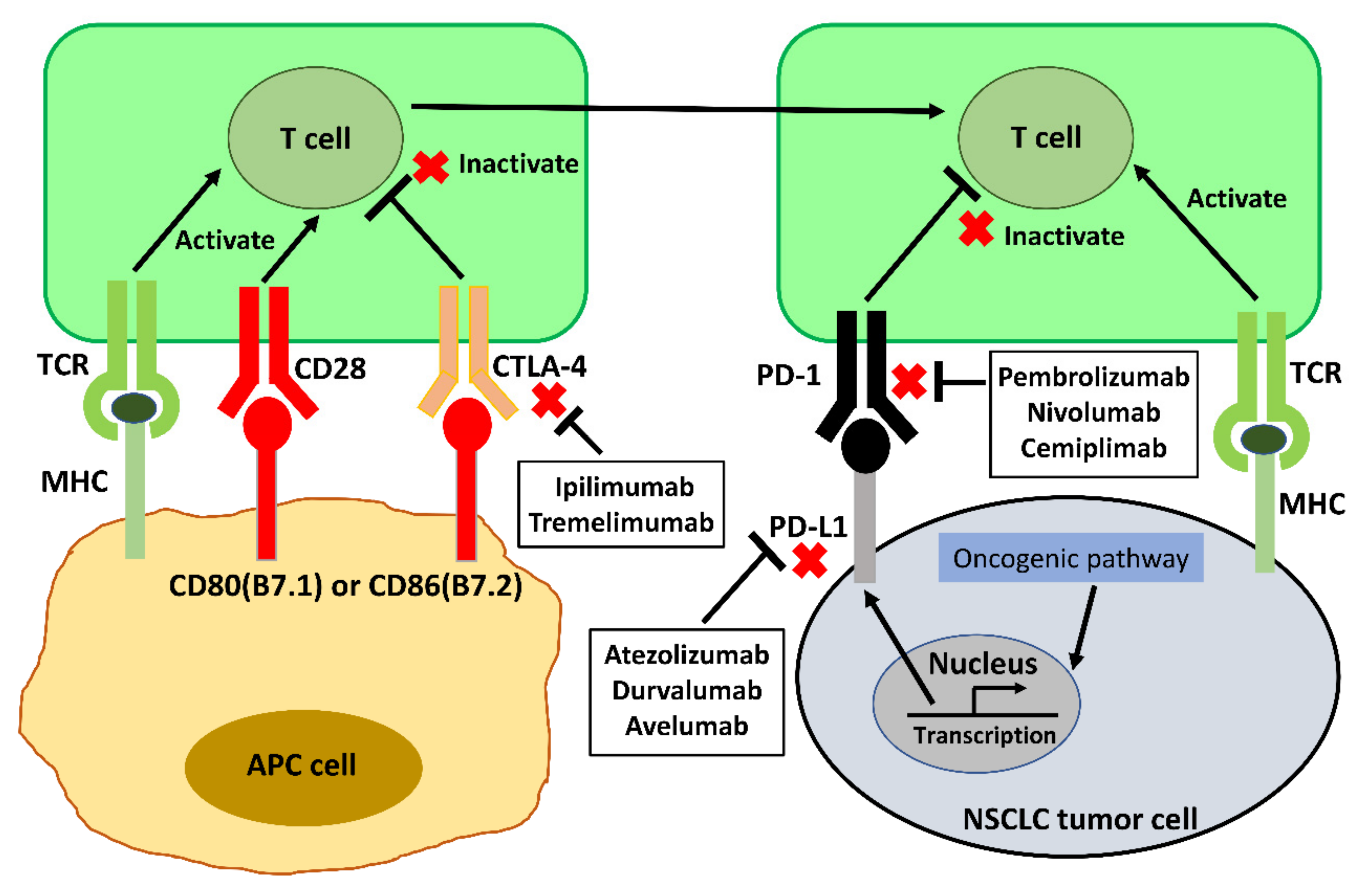
Vaccines Free Full Text Immunotherapy And Vaccination In Surgically Resectable Non Small Cell Lung Cancer Nsclc Html

Personalized Cancer Vaccination In Head And Neck Cancer Shibata 2021 Cancer Science Wiley Online Library
Post a Comment
Post a Comment